Devices and Oral Appliances to Treat Sleep Apnea
Reviewed by: HU Medical Review Board | Last reviewed: May 2025 | Last updated: January 2026
Sleep apnea is a sleep disorder in which a person has shallow breathing or stops breathing for several seconds or longer during sleep. These people tend to snore loudly or snort, gasp, or choke while asleep. Many devices can be used to treat sleep apnea. All are designed to get more air into the lungs and increase oxygen levels in the bloodstream.1
Positive airway pressure (PAP) machines are devices most often used. There are several types of PAP machines. Other treatment options include dental devices and hypoglossal nerve stimulation.
CPAP machines for sleep apnea
Continuous positive airway pressure (CPAP) machines work well to treat people with sleep apnea. That is why the American Academy of Sleep Medicine recommends that CPAP (or a similar machine) be prescribed for anyone with obstructive sleep apnea (OSA).2
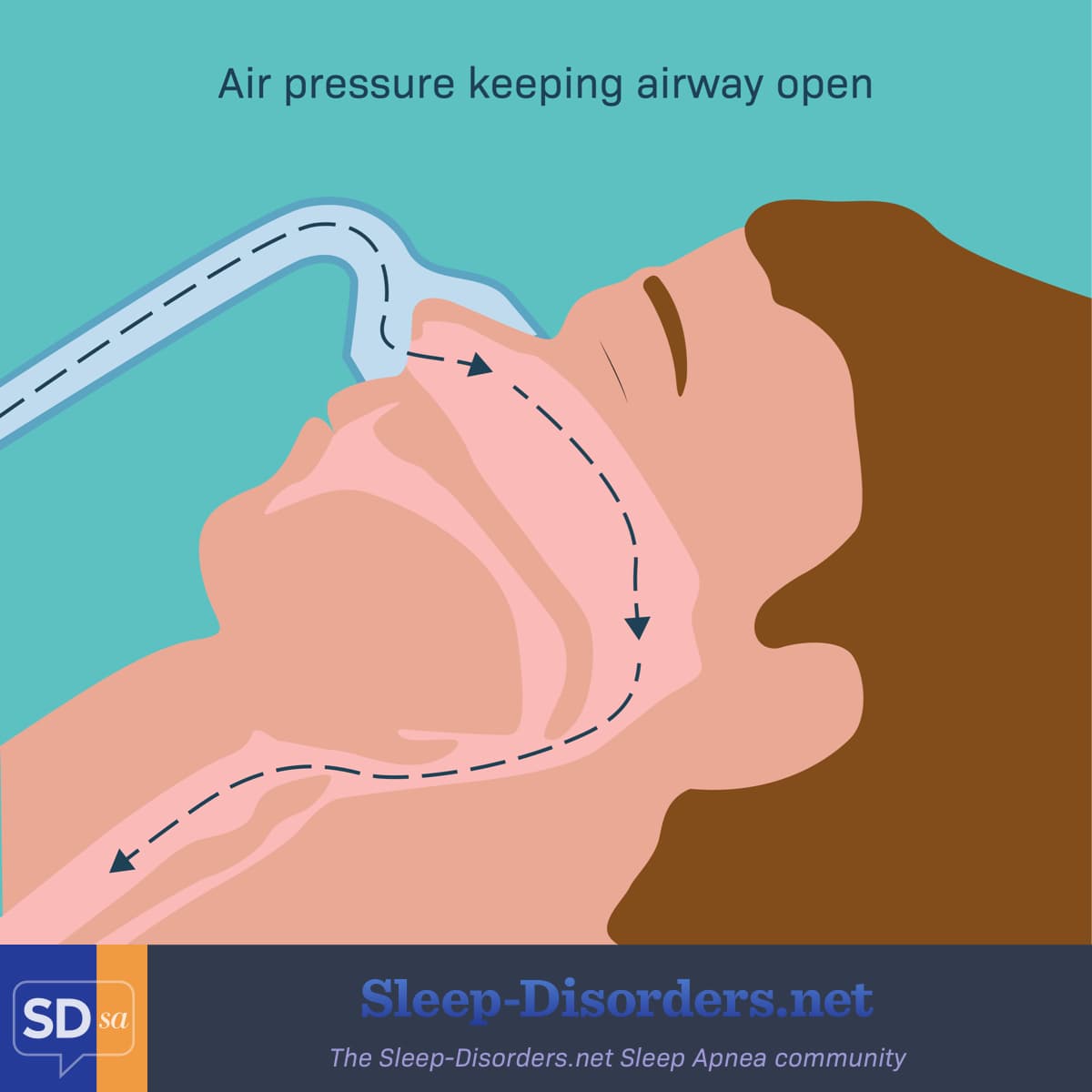
A CPAP machine works by gently forcing pressurized air through a mask and into your airway. The air pressure keeps your airway from collapsing while you sleep. The mask may fit just over the nose, or over the nose and mouth.3
Figure 1. How a CPAP keeps the airway open
It can take some time to get used to sleeping with a CPAP machine. One in every 3 people finds it hard to sleep with a CPAP machine and eventually quits using it. About half of people quit using their CPAP machine over time or only use it for half the night.2,3
However, studies show that when used correctly and consistently, CPAP machines help improve health because your body gets enough oxygen during sleep. This increases sleep quality and daytime functioning. These machines also help reduce daytime sleepiness, blood pressure, the number of vehicle accidents, and depression.2
A few studies suggest that CPAP therapy may improve symptoms of gastroesophageal reflux (GERD), heart failure, atrial fibrillation, and arrhythmias.2
There are other types of positive airway pressure machines, but CPAP is the one most often used.2,4
BiPAP, APAP, and EPAP for sleep apnea
A CPAP machine delivers the same amount of air when you inhale and exhale. BiPAP stands for bilevel positive airway pressure. BiPAP machines deliver higher pressure air on the inhale and lower pressure air on the exhale. BiPAP machines may be an option for people who have trouble exhaling when using a CPAP machine.3
APAP stands for auto-adjusting positive airway pressure. An APAP machine changes pressure throughout the night depending on what is needed to keep your airway open. This pressure changes depending on your sleep position or the stage of sleep you are in.3
EPAP stands for expiratory positive airway pressure. These prescription devices are inserted into the nostrils and help to keep the nostrils open. They also have a valve that closes when you exhale to create pressure that keeps your airway open. EPAP may be an option for people who cannot sleep with a CPAP machine or people who want to travel without their CPAP.3
Dental and tongue devices for sleep apnea
Dental, or oral, appliances may be prescribed for people with mild to moderate sleep apnea who do not want to sleep with a CPAP machine. These devices fit in the mouth and keep the airway open during sleep. They work by holding the chin forward or the tongue down. Some also trap air in the airway to help keep it open.4,5
eXciteOSA® is a device that improves how the muscles in the tongue work. The device works by stimulating the tongue muscles so the tongue does not fall backward during sleep. It is used while you are awake. It requires a prescription and is available for people 18 and older who snore or who have mild sleep apnea.7
Oral appliances are not generally recommended for people with severe OSA.4,6,7
Hypoglossal nerve stimulation for sleep apnea
Inspire is a nerve stimulation device that may work for certain people with moderate to severe OSA. The device is implanted under the collarbone during surgery. It stimulates the hypoglossal nerve, which activates the genioglossus muscle and keeps the upper airway open.4
To qualify for Inspire, you must:3
- Struggle with CPAP or be unable to get consistent benefits from it
- Be 18 or older
- Have a body mass index (BMI) below 32 (this varies by insurance payor and can go up to 40)
- Be diagnosed with moderate to severe obstructive sleep apnea (OSA) with an apnea-hypopnea index (AHI) between 15 and 100
- Children with Down syndrome who have severe OSA (AHI 10-50) and have already had their tonsils and adenoids removed
PAP and mission-critical workers
CPAP or one of its variations is highly recommended for anyone working in what is called a mission-critical job. These jobs include airline pilots, airplane controllers, train engineers, bus drivers, truck drivers, and shipmates. This is because CPAP is highly effective and also collects data showing that the driver is using their device and that it is working well.4